|
2006 Update:
Our major new initiative is to obtain monoclonal antibodies to post-translational modifications in native human OPN. Evidence in the literature that post-translational modifications (serine phosphorylations) are important in certain OPN functions coupled with our desire to obtain mAbs capable of inhibiting OPN function in vivo have led us to the conclusion that such mAbs may be useful, for example to suppress osteoporosis, the progression of autoimmune disease, and metastasis. Financial support from the National Multiple Sclerosis Society through our Stanford colleague Dr. Larry Steinman together with a supply of human native milk OPN from our collaborator Dr. Esben Sørensen (Aarhus, Denmark) has made this effort possible. Melissa Weidner, supported by a GH Cook Scholarship and working on her senior thesis, obtained and partially characterized several dozen hybridomas from OPN-null mice immunized with native OPN. Josephine Cassella, an intern from Kean College working on her Masters degree in Biotechnology, together with undergraduate Tanya Gordonov, (an Aresty Summer Fellow) are actively involved in obtaining and characterizing additional hybridomas. Because of the unusual properties of OPN (an unstructured highly negatively charged molecule) we have elected to screen all the hybridomas for the production of anti-OPN mAbs in several different ways (Elisa, western, peptide binding, and a novel fluorescence assay for the binding of the mAb to OPN in solution). The attached poster (Gornonov et al.) summarizes our progress in the summer of 2006.
Graduate student Christian Kazanecki is investigating the relationship between OPN structure and OPN function. He has discovered that OPN produced by osteoblasts and fibroblasts in cell culture differ in post-translational phosphorylations; some of his current studies address the question of whether these two forms have different functional properties. Senior undergraduate Michael Roberts worked closely with Chris on several projects including epitope mapping and nitric oxide production. We have established collaborations with several groups at Rutgers, branching into disciplines outside biology, in an effort to gain a better understanding of OPN's function in the bone environment and the immune system. In collaborations with our colleagues in Materials Science and Engineering (Drs. Richard Riman and Adrian Mann) Chris is participating in a variety of studies. With graduate student Dan Haders (shown here, with Chris) for example, to elucidate aspects of the interactions of bone cells and OPN with hydroxyapatite, particularly from the point of view of how OPN influences mineral formation. With Christina Sever we are investigating the growth of osteoblasts and endothelial cells in a demineralized bone matrix. The poster by Beril Kavukcuoglu et al. describes some of the preliminary studies on the nanomechanics of OPN-deficient bones. We also participate with our colleague Dr. Joachim Kohn in the Center for Biomaterials Research to characterize the biological properties of novel synthetic materials that might serve as implants in particular contexts. The poster by Milca Aponte-Roman et al. reports some initial studies on osteoblast growth and mineral formation on these novel polymers. The goal of our studies is to improve our understanding of how to design bone implants so that they will integrate more effectively with living tissue. Graduate student Kathryn Wang (shown here with David Denhardt) has undertaken, in collaboration with our colleague Yufang Shi (Robert Wood Johnson Medical School, University of Medicine and Dentistry of New Jersey - RWJMS, UMDNJ), to investigate how OPN mediates the response of the immune system to stress (see poster by Roberts et al.). During the summer of 2006 we have been fortunate to host in our laboratory two graduate students, Lotte Schack and Brian Christiansen, from Dr. Sørensen’s Aarhus University laboratory. Brian is working with Chris on the OPN structure-function relationship, and Lotte is investigating aspects of OPN function in the immune system in collaboration with our colleague Dr. Yacov Ron (RWJMS, UMDNJ). Studies include investigations of the role of OPN in B and T cell proliferation.
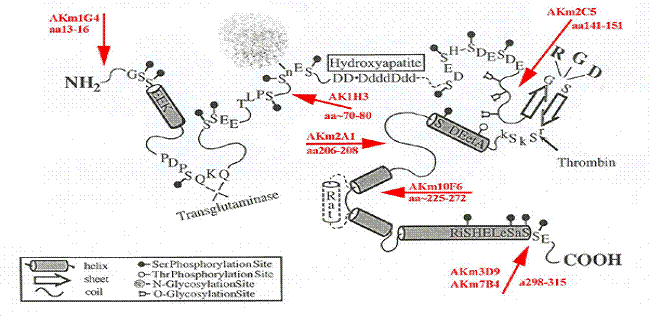
Two undergraduate students, Jennifer Luo and Bhumika Desai, have initiated studies on what integrins mediate OPN induction of MMP expression (Jen) and which anti-OPN monoclonal antibodies recognize the form of OPN found in mouse serum (Bhumie). Research on TIMP has continued in the capable hands of Maya Kappil, a Henry Rutgers Scholar, and, more recently, Gurbir Johal (Sunny). Maya’s contributions are in part reported in our 2005 and 2006 publications (Porter et al., 2005; Khan et al., 2006). Ongoing studies, in part with our colleague Wendie Cohick, have been pursued by Sunny, who is characterizing changes in secreted protein patterns and microRNAs in cells exposed to TIMP. Research in the Denhardt Laboratory: Summary of recently completed and ongoing projects 1. Osteopontin (OPN) signaling: The goal of this research is to shed light on the signaling pathways stimulated by OPN. For a review on OPN see Denhardt et al., J Clin Invest. 107:1055, 2001. We know that in various paradigms OPN can inhibit the apoptotic response, presumably the result of OPN-receptor interactions that initiate signals that suppress the apoptotic response. Our current studies suggest that OPN can either inhibit the activation of anti-apoptotic members of the Bcl-2 family or stimulate the activity of the pro-apoptotic Bax family, or both (Khan et al., J. Cellul Biochem. 85:728, 2002). Subarna Khan, in collaboration with our colleagues in Canada (Ann Chambers, Alan Tuck and Amy Cook) has recently shown that OPN regulates CD44 expression, increasing the abundance of the standard form (Khan, Cook, Kappil, Gunthert, Chambers, Tuck, Denhardt (2006) Enhanced cell surface CD44 variant (v6, v9) expression by osteopontin in breast cancer epithelial cells facilitates tumor cell migration: Novel post-transcriptional, post-translational regulation. Clin Exp Metastasis. May 12; [Epub ahead of print] . 2. Osteopontin structure: Monoclonal antibodies to OPN have been generated by Aaron Kowalski using our OPN-deficient mice; they are currently being characterized with regard to the epitope they recognize and their usefulness in different applications – for example, can they inhibit OPN signaling? These different monoclonals will be used in various studies to characterize post-translational modifications of the protein and functional receptor-OPN interactions (D’Alonzo et al., J Biol Chem 277:24788, 2002). Because OPN can stimulate bone resorption (Yoshitake et al., PNAS 96:8156, 1999; Ihara et al., J Biol Chem. 276:13065, 2001) and autoimmune processes (Chabas et al., Science 294:1731, 2001; Yumoto et al., PNAS 99:4556, 2002), studies are underway to determine if any of these monoclonal antibodies will inhibit stress-induced bone remodeling or suppress autoimmune disease progression. 3. Osteopontin function: We are studying cells isolated from the bones of mice unable to synthesize OPN, and have presented evidence that OPN participates in the sensing of fluid flow by bone cells (Denhardt et al., BBRC 288:448, 2001). The bone cells are grown from fragments of the long bones of wildtype and OPN-null mice. When cultured on slides and exposed to pulsatile fluid flow, the OPN-deficient cells exhibit an altered response, compared to control wildtype cells, as assessed by NO and PGE2 production. More recently, Christian Kazanecki has discovered that osteoblasts unable to make OPN exhibit interesting deficits in their ability to form mineral. He is presently characterizing how two forms of OPN that differ in their post-translational modifications affect the mineralization process using immortalized lines of OPN-/- osteoblasts. Using Osteologic discs, Tanupriya Contractor has found that OPN does not seem to enhance the action of osteoclasts in the resorption of this collagen-free mineral. A major goal was to identify the monoclonal that was most effective at inhibiting osteoclast function (Contractor, Babiarz, Kowalski, Rittling, Sorensen, Denhardt (2005) Osteoclasts resorb protein-free mineral (Osteologic discs) efficiently in the absence of osteopontin. In Vivo19:335-41). 4. OPN expression: OPN is expressed at high levels in cells with an activated Ras oncogene. We have reported the existence of a Ras-activated element (RAE) in the promoter of the OPN gene and presented evidence that in many metastatic cell lines a protein, a Ras response factor (RRF), that binds to this element is present at elevated levels (Denhardt et al., J Expt Metastasis, 20:77, 2003). In collaborative studies with Dr. Quynh Le and colleagues at Stanford, we have investigated whether the RRF/RAE interaction contributes to increased OPN expression in various inflammatory situations, which often involve Ras activation (Zhu, Denhardt, Cao, Sutphin, Koong, Giaccia, Le (2005) Hypoxia upregulates osteopontin expression in NIH-3T3 cells via a Ras-activated enhancer Oncogene24:6555-63). 5. Tissue inhibitor of metalloproteinases-1 (TIMP-1) inhibits the activity of enzymes known as metalloproteinases (MPs). These enzymes degrade components of the extracellular matrix and cell surface proteins. TIMP-1 can stimulate various cellular activities including DNA synthesis. Joseph Porter has obtained evidence that TIMP-1 stimulates cells by inhibiting a constitutively active cell surface metalloproteinase that cleaves regulatory proteins at the cell surface (Porter et al., Brit J Cancer 90:463, 2004). More recently in an oligonucleotide microarray study he has identified genes that are regulated by TIMP-1. (Porter et al., 2005, Breast Cancer Res Treat. 94:185-93. ). Current research is focused on the identity of the cell surface metalloproteinases, possibly an ADAM, and its substrate. We are also investigating whether exposure to TIMPs can modulate the intracellular microRNA levels. 6. We have recently begun an extensive effort to isolate and characterize new monoclonal antibodies raised against native human (milk) OPN. What follows is a fairly extensive description of this endeavor with some background. OPN is both a cytokine, found in all body fluids, and a cell adhesion protein, found in mineralized tissues. Intracellular forms (localized to the inner surface of the plasma membrane and possibly the nucleus) have also been described in certain cells. OPN is an open, flexible molecule, in part because of the abundance of negative charges resulting from the many acidic amino acids and multiple phosphoserines (a post-translational modification) distributed along its length. Constitutively produced by a number of cell types (e.g. osteoblasts, osteoclasts, activated macrophages and T cells, inflamed epithelia), its expression is enhanced in many pathologies. OPN is known to interact with certain integrins and CD44 variants, both widely expressed cell surface receptors. As a consequence of its potential to interact with multiple and ubiquitously expressed cell surface receptors, the intracellular signaling pathways that are activated depend on the particular receptors that are engaged. One common thread linking many of the experimental observations is that OPN helps the mammalian cell survive potentially lethal insults, e.g. hypoxia or growth factor deprivation. OPN has multiple functions in the mammalian organism. It is required for bone remodeling: In the absence of OPN, bone loss resulting from estrogen deprivation (osteoporosis) or from hind-limb unloading (tail-suspension) is much reduced. The progression of autoimmune disease (experimental allergic encephalomyelitis, a mouse model of multiple sclerosis, and rheumatoid arthritis) is attenuated in the absence of OPN. We hypothesize that a monoclonal antibody able to suppress the ability of OPN to function in either of these processes would have potential therapeutic usefulness in suppressing OPN-dependent bone loss and autoimmune disease progression resulting from various pathologies. OPN is secreted into extracellular fluids and is highly conserved among vertebrates. Thus specific high-affinity anti-OPN antisera have been difficult to develop because wild type mice are tolerant of the protein. To get around this block to making effective anti-OPN MAbs, we have exploited our OPN-null mice, mice we developed here at Rutgers some 10 years ago (Rittling et al., 1998), to make antibodies; because they do not make OPN, they “see” OPN as a foreign protein and thus make antibodies against it. Some dozen of several dozen monoclonals, made from mice immunized with recombinant (unmodified) OPN, have been characterized in our laboratory. The amino acid sequence (the epitope) recognized by six of the MAbs has been determined using an OPN gene fragment library displayed on the surface of T7 phage (Kowalski, 2005, PhD thesis, Rutgers University). The locations of the epitopes that we have determined so far are indicated on Fig. 1. We have found that four of the monoclonals recognize epitopes that are often blocked by phosphorylation or glycosylation in the native protein. Fig. 1 shows a schematic of the human OPN molecule with landmark regions noted, including the approximate binding locations for the seven monoclonal antibodies for which the epitope has already been mapped [1G4, 1H3, 2C5, 10F6, 3D9/7B4, and 2A1 (now marketed by Santa Cruz Biotechnology as sc21742. Knowledge of the protein epitope recognized by each antibody will enable us to identify sites of post-translational modification (PTM). OPN contains numerous PTMs, but their functional significance is unclear. Our recent discovery (Kazanecki, unpublished) that 2C5, 3D9, 1H3, and 10F6 are able to bind to OPN produced by fibroblasts, but not by osteoblasts or mammary epithelial cells, suggests that these latter cells modify (by glycosylation or phosphorylation) the target epitopes so that they cannot be recognized by the antibody; the epitopes recognized by 2A1 and 1G4 seem not to be subject to PTM (Kowalski, 2005; Kazanecki, Kowalski and Denhardt, in preparation).
Fig. 1: Schematic of the human OPN protein [modified from Sodek et al., 2000]. The locations of the epitopes recognized by monoclonal antibodies that have been characterized to date are indicated by the large arrows. 3D9 and 7B4 appear to recognize the same epitope (Kowalski, 2005). These monoclonals were all raised against recombinant (unmodified) human and mouse OPN. Recent evidence indicates that phosphorylation of serine residues in the epitopes recognized by 1H3 and 3D9/7B4 prevents the binding of the antibody to the native post-translationally modified protein. RGD is the integrin binding site. A conserved thrombin cleavage site and the aspartate-rich hydroxyapatite binding site are noted as is the presence of an extra exon in the rat protein. One of the conclusions of Aaron Kowalski's research was that we needed a greater selection of monoclonal antibodies; particularly ones against post-translational modifications (phosphorylated regions in particular). We have therefore developed a new strategy (described below) to obtain a more complete set of monoclonals. This novel strategy for screening hybridomas is intended to allow the identification of monoclonal antibodies reacting with any portion of the OPN molecule. The earlier strategy, the one commonly used, requires the adsorption of the protein antigen to a plastic surface (typically the wells of a 96-well plate). This is known as an ELISA (enzyme-linked immunosorbent assay). We believe this restricts the types of MAbs that can be found because portions of the protein are bound to the plastic, sequestering epitopes that would otherwise bind monoclonals targeting that region. Similar, OPN bound to a membrane (PVDF, nitrocellulose) might not efficiently display certain epitopes. Hybridomas potentially producing anti-OPN monoclonals have been isolated in the conventional way – fusing spleen cells from an OPN-null mouse immunized with OPN with an immortal myeloma line and then selecting for cells that retain the immortality of the myeloma line and the antibody-producing capability of the mortal spleen cell. The hybrid line thus derived (as the result of a drug-[“HAT”]selection procedure) is the hybridoma, and each one is capable of producing only one particular kind of antibody that recognizes a single unique epitope – hence “monoclonal”. The immunogen, highly purified human milk OPN, was generously provided by our Danish collaborator, Dr. Esben Sørensen (Tian et al., 2000; Contractor et al., 2005; Christensen et al., 2005). Recombinant human OPN is produced in our laboratory from bacterial cells engineered to produce human OPN (Rollo et al., 1996). To identify anti-OPN MAbs during the screening of the hybridomas, we are using a differential fluorescence procedure to distinguish those monoclonals able to bind a post-translationally modified epitope from those monoclonals binding epitopes found in the polypeptide backbone itself. To detect binding of individual monoclonals (shown to bind recombinant protein) to specific peptides: A set of 19 overlapping peptides covering almost the entire OPN protein sequence is being used to identify the epitopes recognized by our different monoclonals. Each of these peptides has a biotin molecule bound to the C-terminus of the peptide via a linker consisting of two glycine residues. The biotin enables the strong binding of the peptide to the surface of a streptavidin-coated 96-well plate. The uncharacterized monoclonal antibody is allowed to react with the different peptides in the wells of a 96-well plate; after the wells are washed, bound antibody is detected with a goat anti-mouse IgG conjugated with a fluorescent tag (Alexa Fluor 594). This form of epitope mapping will allow us to immediately classify the monoclonals according to the approximate location of the recognized epitope. Functional assays: We have had considerable experience with the use of OPN to inhibit the induction of inducible nitric oxide synthase (iNOS) (Hwang et al., 1995; Rollo et al., 1996; Tian et al., 1999). During inflammatory processes, agents such as bacterial lipopolysaccharide (LPS) and interferon gamma (IFNγ) induce the transcription of the iNOS gene, and the consequent elevated expression of NO has a wide-ranging and substantial impact on the inflammatory response. The ability of OPN to modulate NO production by, for example, macrophages is important in physiological processes (Tian et al., 1999). Very recently, graduate student Kathryn Wang has discovered a dramatic effect of OPN on the response of mice to stress (hind-limb unloading). We are anxious to test the ability of our monoclonal antibodies to suppress the response of the wild-type mouse to this stress, effectively converting the wild-type phenotype into the OPN-null phenotype. These monoclonals would then be promising candidates to suppress osteoporosis and/or autoimmune disease progression in the wild-type mouse (or human). Rationale and Significance: It is well established that OPN plays important but poorly understood roles in numerous inflammatory pathologies; relevant in the present case are osteoporosis, stress-induced bone remodeling, cancer metastasis, and autoimmune disease (reviews: Denhardt and Noda, 1998; Sodek et al., 2000; Denhardt et al., 2001). Knowledge gained from research with inhibitory monoclonal antibodies will add substantially to our understanding of how OPN regulates these various aspects of mammalian systems physiology. OPN appears uniquely capable, as a soluble cytokine, to deliver signals to cells similar to those generated when cells interact with extracellular matrix components. Bone remodeling, a complex process that involves both osteoblasts and osteoclasts, relies intimately on OPN. OPN serves both as an attachment factor and cytokine for osteoclasts, promoting their motility and survival; osteoclasts unable to make OPN do not resorb bone efficiently. Changes in gene expression as osteoblasts differentiate from a pre-osteoblastic state to the mature mineralizing osteoblast underlie and implement the mineral formation process, a process influenced by the presence of OPN. As noted above, OPN stimulates autoimmune disease progression and cancer metastasis; any compound able to inhibit either of these pathologies may become a clinically useful tool in treating these diseases.
|