|
2008 Update:
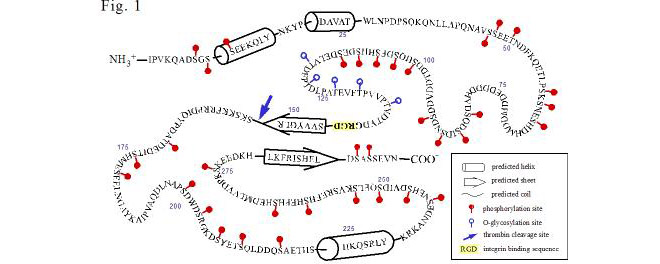
The past two years (2006-7) have seen our research culminate in four publications that report these studies, which were also presented in three posters and two talks presented at the Gordon Research Conference on Small Integrin-binding Proteins held in August, 2007 (Biddeford, Maine). Christian Kazanecki, who recently completed his PhD thesis and has joined Ethicon, a J&J affiliate, showed that two structurally different forms of OPN differ in how each signals cells. OPN produced by osteoblasts is much more highly modified (phosphorylated on serine) than OPN produced by ras-transformed fibroblasts (Poster 1, Kazanecki et al., 2007a; Christensen et al., 2007). FbOPN contains approximately four phosphate groups distributed over 17 potential phosphorylation sites, whereas ObOPN contains approximately 21 phosphate groups distributed over 27 sites. Five residues were O-glycosylated in both isoforms. The two proteins were compared in adhesion assays for their ability to support attachment of human MDA-MB-435 tumor cells and mouse ras-transformed 275-3-2 fibroblast cells. FbOPN mediated binding of the MDA-MD-435 cells with 50% higher efficiency than ObOPN. The opposite trend was observed for the mouse fibroblast line where ObOPN promoted approximately 30% higher binding efficiency than FbOPN. Molecular modeling by Dana Uzwiak has suggested an interesting structural feature (a ß-sheet fold) that could permit the C-terminal end of the OPN molecule to regulate integrin binding at a central RGD sequence via a CD44 interaction (Fig 1; Kazanecki et al., 2007b). In another study, PhD student Kathryn Wang has shown that OPN confers on mice a sensitivity to certain forms of stress (hindlimb unloading, periodic restraint) as evidenced by corticosteroid-mediated atrophy of the spleen and/or thymus (Poster 2; Wang et al., 2007). We hypothesize that OPN is necessary for the stress-induced elevation of corticosterone expression; Henry Rutgers Honors student Jennifer Luo is investigating by RT-QPCR whether transcription of one or more genes involved in steroid synthesis is OPN-regulated. Katie Jaques, a PhD student with Dr. Gary Merrill, has recently initiated a collaboration with us to investigate whether OPN has a significant role in the response of the mouse heart to reactive oxygen species. Sunanda Baliga, also a student with Gary Merrill, is currently in the lab studying OPN expression in the ischemic hippocampus in the presence and absence of acetaminophen.
Our major goal in the immediate future is to identify and characterize an anti-OPN monoclonal antibody that is a functional inhibitor of OPN action in vivo. Such an antibody may be clinically useful in the treatment of autoimmune disease, cancer metastasis, bone resorption (osteoporosis), and pathological stress responses. We have successfully isolated several dozen new anti-OPN monoclonal antibodies some of which recognize post-translational modifications (PTMs), largely serine phosphorylations, in the protein. To identify the epitopes recognized by the different monoclonal antibodies we have employed two strategies. One utilizes peptides corresponding to conserved sequences in the OPN protein that are either phosphorylated or unphosphorylated. Undergraduate Tanya Gordonov is conducting this research, which allows us to determine whether a particular MAb recognizes a specific in OPN. The second strategy employed two different forms of OPN, the highly post-translationally modified native OPN purified from human milk and the unmodified form synthesized in E. coli. As described in their poster, the native form was labeled with the fluorescent tag Oregon Green and the recombinant form labeled with Texas Red. In the novel assay developed during this study by Masters student Josephine Cassella (and continued by undergraduate Cassandra Louis (left, standing), an Aresty Fellow) the two forms were mixed and allowed to react in solution with the monoclonal antibody under investigation. The antigen-antibody complexes were then captured on magnetic beads coupled to protein G (which binds the Fc portion of the IgG). After washing the beads, the adsorbed IgG was eluted at low pH, and the fluorescence at the two wavelengths determined. MAbs that selectively bound the native protein (relative to the modified protein) are presumed to be antibodies that recognize PTMs. This can be confirmed using the peptide assay mentioned above. This new method, which will be reported in a manuscript in preparation) is important in that it detects the antibody-antigen reaction in solution rather than on a solid support, as occurs in the Western and ELISA assays. Undergraduate Yao Li has had major responsibility of the Western Blot analyses. Two first-year undergraduates joined the laboratory in the summer of 2008, Megha Rajpal (an Aresty Summer Fellow) and Jay Panchal. They are participating in the various studies to characterize the anti-OPN monoclonal antibodies (peptide assays, western blots, ELISAs). Megha is also assessing how the mAbs affect growth of cancer cells in soft agar.
Hybridomas with the potential to produce monoclonal antibodies recognizing PTMs in OPN have been prepared in two ways, both of which employed our OPN-null (knockout) mouse. (This is important because OPN is found in the plasma of normal mice, and such mice would be tolerant of the protein and unable to make anti-OPN antibodies very efficiently.) In the “standard” approach the mice were immunized with the highly modified human milk OPN generously provided by our Danish colleague Esben Sørensen. In the alternate approach mice were immunized with a set of five serine-phosphorylated peptides designed to mimic five regions in the OPN protein that are highly conserved between human, mouse, rat and bovine OPN species. Monoclonals derived from these immunizations are currently being tested as described above to determine their specificity (Poster 3). We are now in the process of identifying MAbs that are functional inhibitors of OPN, both in cell culture and in vivo, in the mouse. In cell culture, we have shown that exogenous OPN can inhibit the apoptotic response of human umbilical vein endothelial cells deprived of growth factors (Khan SA, Lopez-Chua CA, Zhang J, Fisher LW, Sørensen ES, Denhardt DT. (2002) Soluble osteopontin inhibits apoptosis of adherent endothelial cells deprived of growth factors. J Cell Biochem. 85:728-36.). In ongoing work, Dana Cifelli, a Masters student who recently joined the lab, is investigating the ability of selected MAbs to suppress the apoptosis-inhibiting action of OPN. In the mouse, Kathryn Wang is continuing her studies described above to determine whether any of our anti-OPN MAbs can block the ability of OPN to cause immune organ atrophy in mice exposed to restraint stress (Poster 3). (We have recently established that it is OPN in the plasma that is necessary for the organ atrophy.) Should this be successful in identifying specific MAbs that inhibit this action of OPN, we would explore their usefulness as compounds that might be clinically useful in the treatment of autoimmune disease, cancer metastasis, and certain forms of stress and/or osteoporosis. We wish to acknowledge the valuable support we obtain from our colleagues and collaborators Yacov Ron, Yufang Shi, Esben Sørensen, and Larry Steinman. Christensen B, Kazanecki CC, Petersen TE, Rittling SR, Denhardt DT, Sørensen ES. (2007) Cell type-specific post-translational modifications of mouse osteopontin are associated with different adhesive properties. J Biol Chem. 282:19463-72. Kazanecki CC, Kowalski AJ, Ding T, Rittling SR, Denhardt DT. (2007a) Characterization of anti-osteopontin monoclonal antibodies: Binding sensitivity to post-translational modifications. J Cell Biochem. 102:925-935 Kazanecki CC, Uzwiak DJ, Denhardt DT. (2007b) Control of osteopontin signaling and function by post-translational phosphorylation and protein folding. J Cell Biochem. 102:912-924. Wang KX, Shi Y, Denhardt DT. (2007) Osteopontin regulates hindlimb-unloading-induced lymphoid organ atrophy and weight loss by modulating corticosteroid production. Proc Natl Acad Sci U S A. 104:14777-82.
|